|
|
| This text is meant to accompany class discussions. It is not everything there is to know about uniform circular motion. It is meant as a prep for class. More detailed notes and examples are given in the class notes, presentations, and demonstrations. See the links below. |
Click here for reading questions.
|
| Review |
| |

When electromagnetic radiation, light, shines on a metal, electrons will be released, if the photon's energy of the light is high enough. When a photon of light is ejected from a source it has an amount of energy directly proportional to the frequency.

If the energy is high enough then the an electron is emitted. If the energy is not high enough, the light will bounce of the surface with the same amount of energy that it hit the the surface. The threshold of energy needed to just barely liberate an electron from the metal is called the work function and is symbolized with the Greek letter, Φ, pronounced ("fee,)" and is measured in electronvolts, eV's. All metals do not have the same work function. In fact the type of metal can be identified by their work functions. The table below is from Wikipedia (accessed 04/15/2016, https://en.wikipedia.org/wiki/Work_function.)
|
|
If the energy of a incident photon is equal to the work function, then an electron will be emitted from the metal. This electron is called a, "photoelectron." It is a regular electron with a special name.
If the energy of the incident photon is larger than the work function then the law of conservation of energy dictates that this extra energy must be accounted for. This extra energy is kinetic energy of the photoelectron. This give the following equation.

Because these energies are so small they are typically measured in electronvolts, eV's, when using this equation. If a photon hits the metal with 5 Ev's of energy and the metals work function is 3 eV's, the an electron is ejected from the metal with 2 eV's of energy. If the intensity of the incident light is increased then more photons will impact the metal and more electrons will be ejected. Recall that electrical current is the amount of charges that flow every second. More ejected electrons means a directly proportional rise in the circuit's current. each electron wil travel with teh same kinetic energy but there will be more of them. If the frequency is increased then the energy will also be increased. Since the work function is a constant, then the extra energy will go into ejecting the electrons with more kinetic energy.
| Example Application |
A "mylar"balloon" is a balloon made from a thin conducting material. You can charge a mylar balloon by physically placing charges on the balloon by rubbing them off of your body to the balloon. To remove the charges you could aim a light of the right frequency at the balloon and the negative charges would be released as photoelectrons. If the Mylar's work function was 3.0 eV's, then what region of the electromagnetic spectrum would release the electrons?
A "mylar"balloon" is a a balloon made from a thin conducting material. You can charge a mylar balloon by physically placing charges on the balloon by rubbing them off of your body to the balloon. To remove the charges you could aim a light of the right frequency at the balloon and the negative charges would be released as photoelectrons. If the Mylar's work function was 3.0 eV's, then what region of the electromagnetic spectrum would release the electrons?
Solution:
Strategy
- Convert 3eV's to Joules.
- Convert to a frequency.
- Look up on a table, from the Internet, to see what part of the electromagnetic spectrum this corresponds to.
3eV's = 3(1.6x10-19J) = 4.8x10-19J
E = hf
Therefore, f = E/h = 4.8x10-19J/6.626x10-34Js = 7.24x1014 Hz
This would put it just below ultraviolet light, UV-A starts at ~7.5x1014 Hz. A violet colored, "black light" or "anti-bacteria" lamp, would discharge the electrons. This process of shining "light" on a surface, can never remove positive charges -only negative charges.
|
The amount of energy gained by an electron between the two plate can be calculated from

However, since the particle being discussed is an electron the Voltage on the battery attached to the plates converts directly to the energy of an electron in eV's. Example
3 Volts ~ 3 eV's
11 Volts ~ 11 eV's
This kinetic energy of the electron.
|
| deBroglie's wavelength |
France's Prince Louis-Victor deBroglie reasoned that if light a particle of light can have a wave property, then why can't a wave have particle properties -like momentum. He came up with a relationship between a particle's wavelength and the momentum. The wavelength is called the deBroglie wavelength.
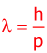
λ:
h:
p: |
wavelength [m] In this equation, the, "λ," is called "deBroglie's wavelength"
Planck's constant ... which is 6.626 x 10-34 m2•kg/s
momentum [s] ...Recall that p=mv |
This is further evidence of the duality between waves and particles.That is a very important connection. Scientists have the tools to measure the wavelengths given off by subatomic particles. But large particles/objects, like a moving baseball, given off wavelengths shorter than a wavelength of visible light and are not detectable with the human eye.
| Example |
What is the deBroglie wavelength for an electro with a kinetic energy of 1000 eV's?
|
|
| Quantum |
The term quantum refers to small discrete steps. If you were to measure you height from the floor as you JUMP up the steps one step at a time.

Your distance from the floor changes by the height of a step, 7 inches. You cannot stop as any height in between as you jump because it is not stable position and you land on the previous step. The quantum world is a world of very, very, very, small sizes and everything occurs in these discrete steps. The rules apply to particles that can behave as waves, like electrons and photons.
How could you make a cruise control on a car such that it's speeds where obviously quantized?
The equation for the energy of a single photon is E=hf. This means the photon of a given frequency can only have energy amounts of (1)hf, 2hf, 3hf, 4hf, etcetera. You cannot have half of a photon, so therefore you cannot have (11/2)hf. The energy associated with electromagnetic radiation is quantized into discrete steps.
|
| Energy Level Diagrams |
Bohr came up with a model for Hydrogen and extrapolated this model for other atoms. His model placed the electrons in orbits around the nucleus. (Today physicists have replaced the Bohr model with probability models. But his model does a good job explaining some phenomena -like light emissions.)
His model also gave us the idea of energy levels. Where each orbit was an energy level. The distance from the nucleus is a measure of the level energy. Below is the energy diagram.
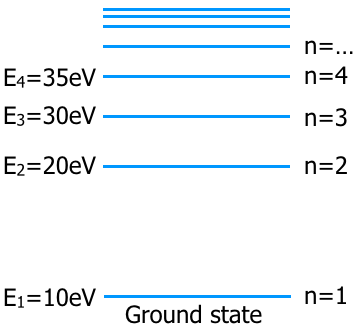
These energy levels correspond to what you learned in chemistry class at the 1s, 2s, 2p, 3s ... orbitals. the energy levels that are shown on the diagram vary from molecule to molecule. The lowest energy state, n=1, would correspond to the the orbital with the smallest radius. The higher orbitals correspond to larger radii.
When a photon with enough energy collides with the electron, the electron will jump to a higher energy state called the excited state. But the photon has to have the exact energy or nothing will happen. For this energy level diagram the photon must have an energy of E2-E1, E3-E1, or E4-E1.

This is called an absorption because the electron absorbs the photon. When a white light shines through a gas, the gas will absorb specific frequencies, or colors, of light. when the light exits the gas and is directed through a prism. Certain colors of the rainbow would be missing because they were absorbed by the gas before the light exited.
The excited state is not stable so the electron will drop back to the ground state. Some electrons will drop directly back to the ground state while others will drop randomly between the levels to get to the ground state. This emission process is called "spontaneous emission." In the digram below the electron took two steps to get back to the ground state.
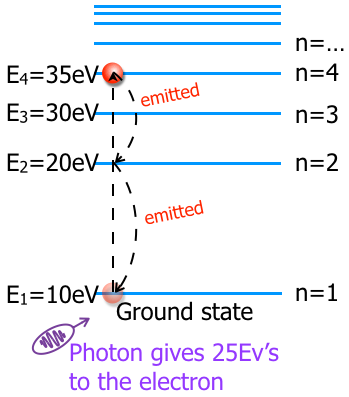
Every time an electron is emitted a photon with an energy equal to the energy level difference is emitted. This creates an emission spectrum. heating or electrifying a gas will create an emission spectrum. A neon light glows with its neon color because those are the visible parts of the electromagnetic spectrum that the energized gas emits.
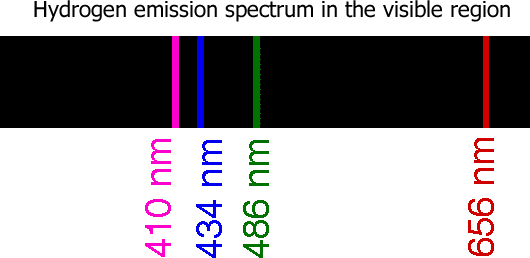
| Example 2 |
Note: This solutions says that I have 7 color emissions. But in the video I counted the n=2 to n=1 energy level change twice. There are 6 spectral lines.
|
|
| When a photon collides with a gas that contains some particles in an excited state, then if the photon's energy is equal to the the energy between the excited state and the ground state, then a photon will be released equal to the energy different between states. This is called stimulated emission. (Because the target particle is already in a stimulated, "higher," energy state.) |
 |
| |
|
|
|